ASC localization and splicing
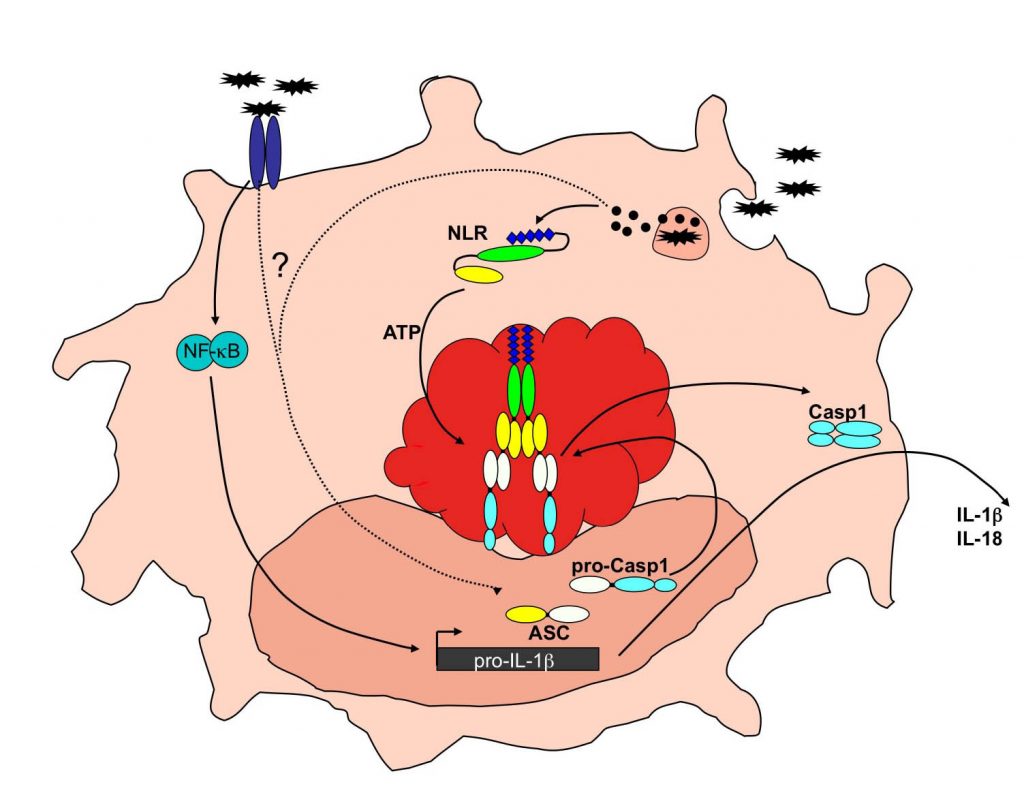
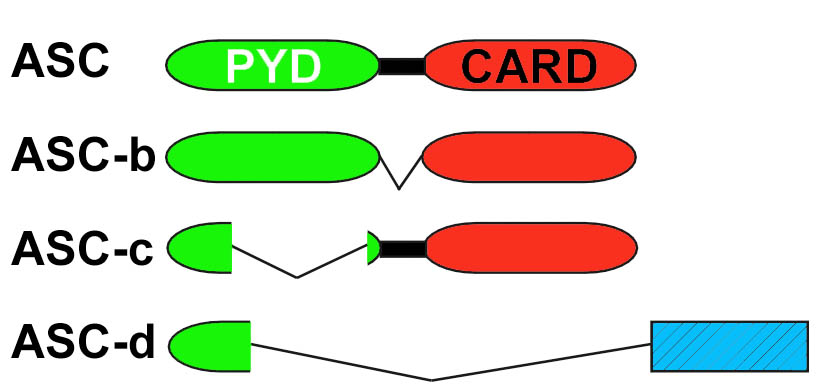
The molecular mechanisms regulating cytosolic PRRs – and in particular inflammasome activity – are still poorly understood. However, since excessive inflammasome activity is directly linked to a wide spectrum of inflammatory diseases, a better understanding of these control mechanisms is likely to provide a basis for the design of novel therapies. We originally co-discovered that ASC is the essential adaptor for caspase-1 [1], and were the first to describe assembly of endogenous inflammasome components into cytosolic aggregates in macrophages and showed that this step requires the inducible redistribution of the inflammasome adaptor from the nucleus to a cytosol during inflammasome priming [2]. We furthermore identified that differential splicing of ASC regulates inflammasome activation by producing proteins lacking key protein-protein interaction domains [3].
References
[1] Stehlik C, Lee SH, Dorfleutner A, Stassinopoulos A, Sagara J, Reed JC. Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. Journal of Immunology 2003, 171, p6154-6163. PMID:14634131 | pdf
[2] Bryan NB, Dorfleutner A, Rojanasakul Y, Stehlik C. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. Journal of Immunology 2009, 182, p3173-3182. PMID:19234215 | pdf
[3] Bryan NB, Dorfleutner A, Kramer SJ, Yun C, Rojanasakul Y, Stehlik C. Differential splicing of the apoptosis-associated speck like protein containing a caspase recruitment domain (ASC) regulates inflammasomes. Journal of Inflammation 2010, 18, p7-23. PMID:20482797 | pdf
PYRIN domain-only proteins (POPs)
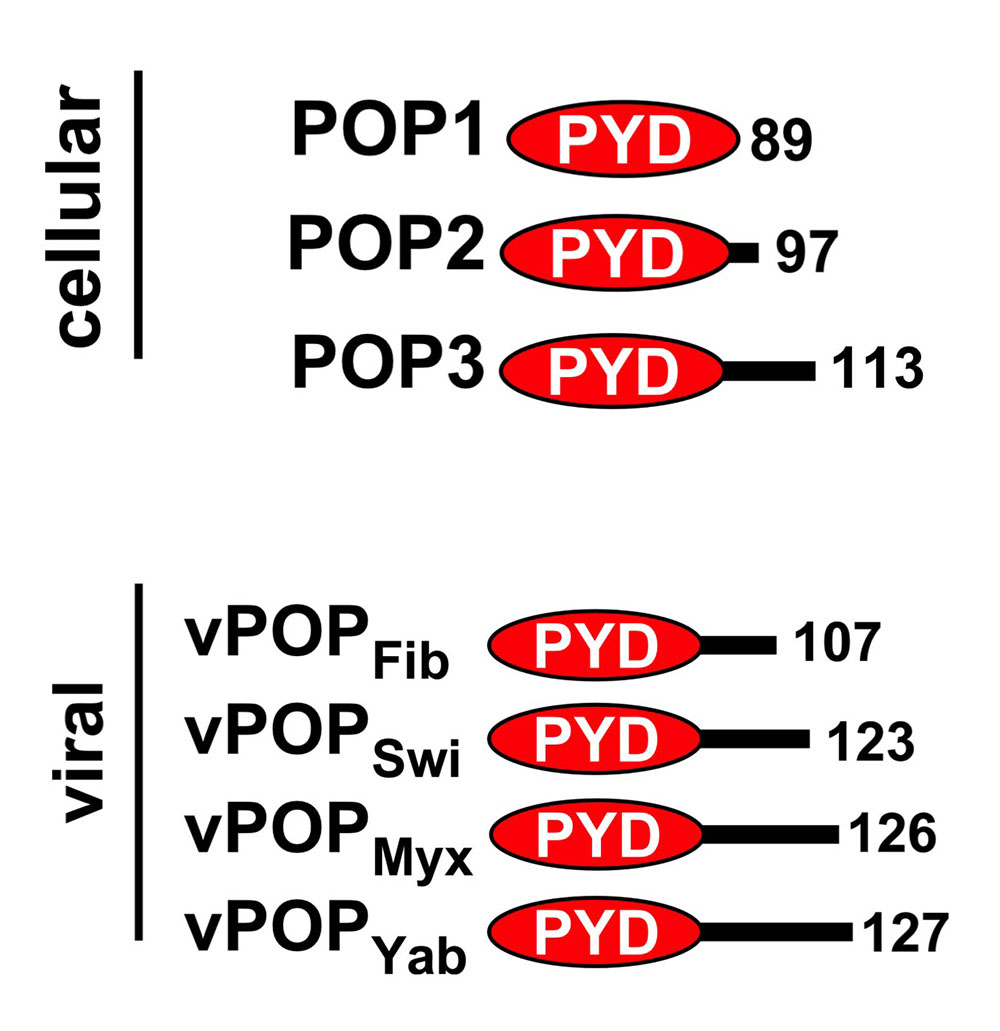
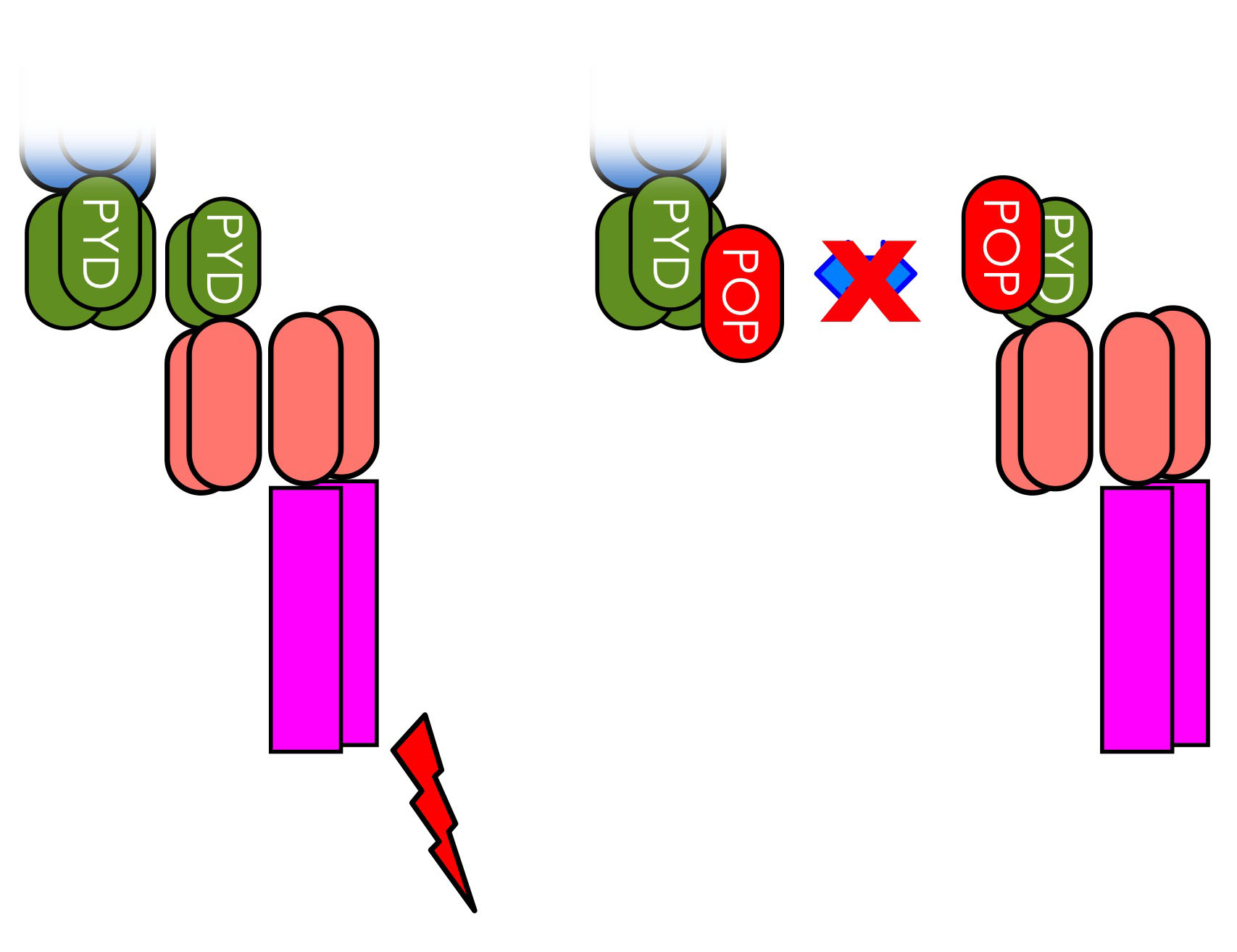
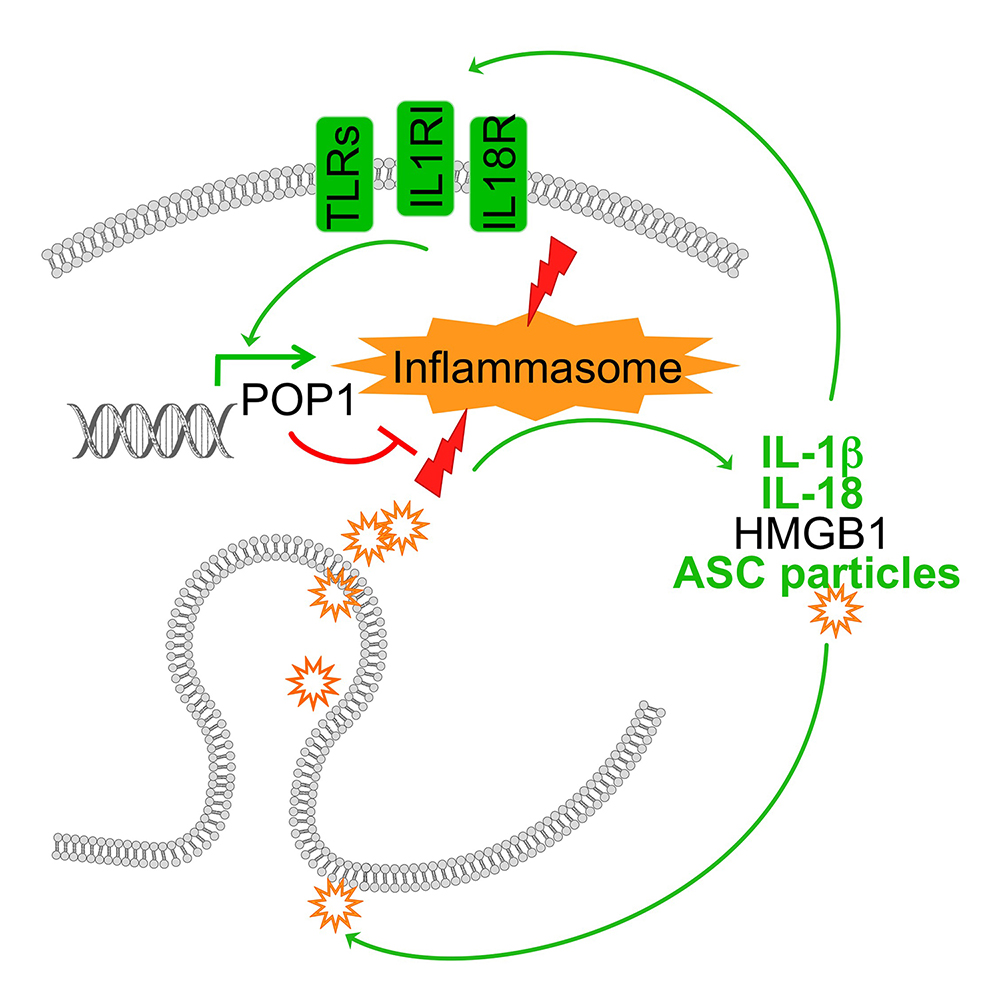
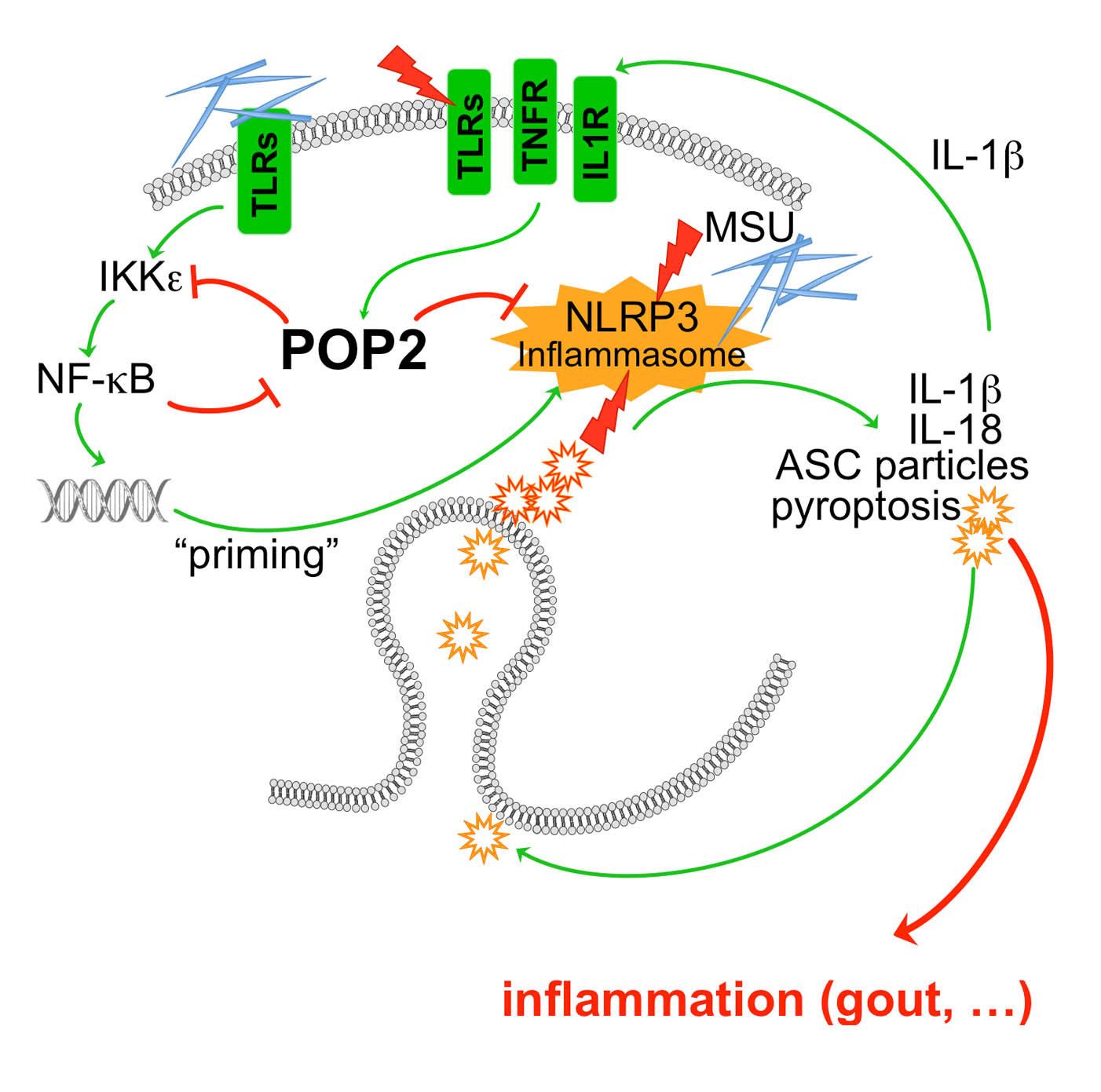
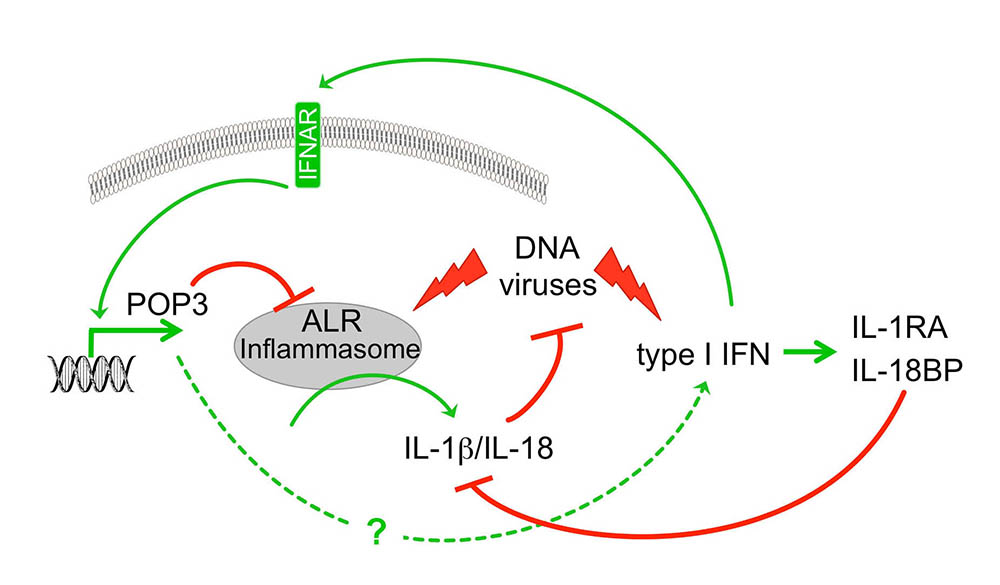
Inflammasome assembly results in nucleation of ASC polymerization by upstream PRRs, which results in clustering of caspase-1 and activation by induced proximity. This step requires interaction between PRRs, ASC and caspase-1 via specific protein-protein interaction domains, namely the PYRIN domain (PYD) and caspase recruitment domain (CARD) (reviewed in [1, 2]). We observed significant species differences in the repertoire of these proteins in humans and mice [3], and discovered and established the family of small, single PYD-containing PYRIN domain-only proteins (POPs) [4], which evolved in humans as inflammasome regulators, but are lacking from other species and may allow a more refined control of inflammasome responses [5]. We discovered and characterized all POP members, including POP1 [4, 5], POP2 [6, 7] and POP3 [8] in humans and discovered also several viral POP family members [9]. We identified that POPs function by binding to the PYD in either ASC or upstream PRRs, occupying this essential protein interaction motif and thereby preventing inflammasome assembly by preventing nucleation of ASC polymerization. Our studies also revealed specificity of individual POPs for particular inflammasomes. As expected from key regulators, expression of POPs is undetectable in resting cells and highly inducible upon inflammatory activation of macrophages.
POP1 likely originated by exon duplication of the ASC-PYD, binds to ASC and prevents recruitment of ASC to NLRP3. Consequently, POP1 prevents ASC polymerization, caspase-1 activation and release of IL-1b and IL-18. POP1 and POP2 bind to ASC and prevents recruitment to activated NLRP3. Consequently, POP1 and POP2 prevent ASC polymerization, caspase-1 activation and release of IL-1b and IL-18 as well as pyroptosis. POP3 on the other hand selectively binds to the PYD of the ALRs AIM2 and IFI16 and prevents DNA and DNA virus-induced inflammasome activation and IL-18 production. POP2 additionally utilizes a second mechanism to inhibit inflammasome responses by targeting inflammasome priming. As expected from key regulators, expression of POPs is undetected in resting cells and highly inducible upon inflammatory activation of macrophages – in the case of POP1 and POP2 by pro-inflammatory cytokines and PAMPs, such as LPS, and DNA and type I interferons in the case of POP3. Hence, POPs function in an inflammasome regulatory feedback loop and are likely involved in the resolution phase of inflammasome responses.
Since POPs are lacking from mice, we generated transgenic mice expressing POPs in the monocyte macrophage lineage and showed that POPs have a role in blocking excessive inflammasome responses in vivo. In particular, POP1 and POP2 are capable of efficiently ameliorating inflammatory disease induced by PAMPs, such as LPS, by danger signals such as MSU crystals and auto inflammatory disease triggered by mutations in inflammasome components, such as NLRP3 mutations in Cryopyrinopathy.
References
[1] Chu LH, Gangopadhyay A, Dorfleutner A*, Stehlik C*. An updated view on the structure and function of PYRIN domains. Apoptosis 2015, 20, p157-173. PMID:25451010 | pdf
[2] Dorfleutner A*, Chu L, Stehlik C*. Inhibiting the inflammasome: one domain at a time. Immunology Reviews 2015, 265, p205-216. PMID:25879295 | pdf
[3]Reed JC, Doctor K, Rojas A, Zapata JM, Stehlik C, Fiorentino L, Damiano J, Roth W, Matsuzawa S, Newman R, Takayama S, Marusawa H, Xu F, Salvesen G, Godzik A; RIKEN GER Group.; GSL Members. Comparative analysis of apoptosis and inflammation genes of mice and humans. Genome Research 2003, 13, p1376-1388. PMID:12819136 | pdf
[4] Stehlik C, Krajewska M, Welsh K, Krajewski S, Godzik A, Reed JC. The PAAD/PYRIN-only protein POP1/ASC2 is a modulator of ASC-mediated nuclear-factor-kappa B and pro-caspase-1 regulation. Biochemical Journal 2003, 373, p101-113. PMID:12656673 |pdf
[5] Stehlik C, Dorfleutner A. COPs and POPs: modulators of inflammasome activity. Journal of Immunology 2007, 179, p7993-7998. PMID:18056338 | pdf
[6] de Almeida L, Khare S, Misharin AV, Patel R, Ratsimandresy RA, Wallin MC, Perlman H, Greaves DR, Hoffman HM, Dorfleutner A*, Stehlik C*. The PYRIN Domain-only Protein POP1 Inhibits Inflammasome Assembly and Ameliorates Inflammatory Disease. Immunity 2015, 43, p264-276. PMID:26275995 | pdf
[7] Dorfleutner A, Bryan NB, Talbott SJ, Funya KN, Rellick SL, Reed JC, Shi X, Rojanasakul Y, Flynn DC, Stehlik C. Cellular pyrin domain-only protein 2 is a candidate regulator of inflammasome activation. Infection and Immunity 2007, 75, p1484-1492. PMID:17178784 | pdf
[8] Ratsimandresy RA, Chu LH, Khare S, de Almeida L, Gangopadhyay A, Indramohan M, Misharin AV, Greaves DR, Perlman H, Dorfleutner A*, Stehlik C*. The PYRIN domain-only protein POP2 inhibits inflammasome priming and activation. Nature Communications 2017, 8, p15556. PMID:28580931 | pdf
[9] Khare S, Ratsimandresy RA, de Almeida L, Cuda CM, Rellick SL, Misharin AV, Wallin MC, Gangopadhyay A, Forte E, Gottwein E, Perlman H, Reed JC, Greaves DR, Dorfleutner A*, Stehlik C*. The PYRIN domain-only rotein POP3 inhibits ALR inflammasomes and regulates responses to infection with DNA viruses. Nature Immunology 2014, 15, p343-353. PMID:24531343 | pdf
[10] Dorfleutner A, Talbott SJ, Bryan NB, Funya KN, Rellick SL, Reed JC, Shi X, Rojanasakul Y, Flynn DC, Stehlik C. A Shope Fibroma virus PYRIN-only protein modulates the host immune response. Virus Genes 2007, 35, p685-694. PMID:17676277 | pdf